Precision medicine, powered by AI, is redefining how drugs are discovered, trials are conducted, and patients are treated. While the FDA doesn’t formally use the term ‘precision medicine’ in its categorization, analyses of approved new molecular entities (NMEs) as targeted therapies based on specific genetic, biomarker, or molecular profiles align with the narrative. This trend is fueling the ongoing momentum and rapid growth of precision medicine in drug development and patient care.
Projections indicate that by 2035, around 50% of all approvals could be precision-grade (see Exhibit 1). The numbers make the shift clear: precision therapies accounted for only 3.7% of drug approvals in 2000 and surged to 32% in 2024, a near tenfold increase. This isn’t just a minor trend; it represents the future of pharmaceutical pipelines.
If you’re leading R&D or digital transformation in pharma, your mandate is clear: build the AI, data, and clinical infrastructure for precision medicine or risk losing relevance in the next growth cycle.
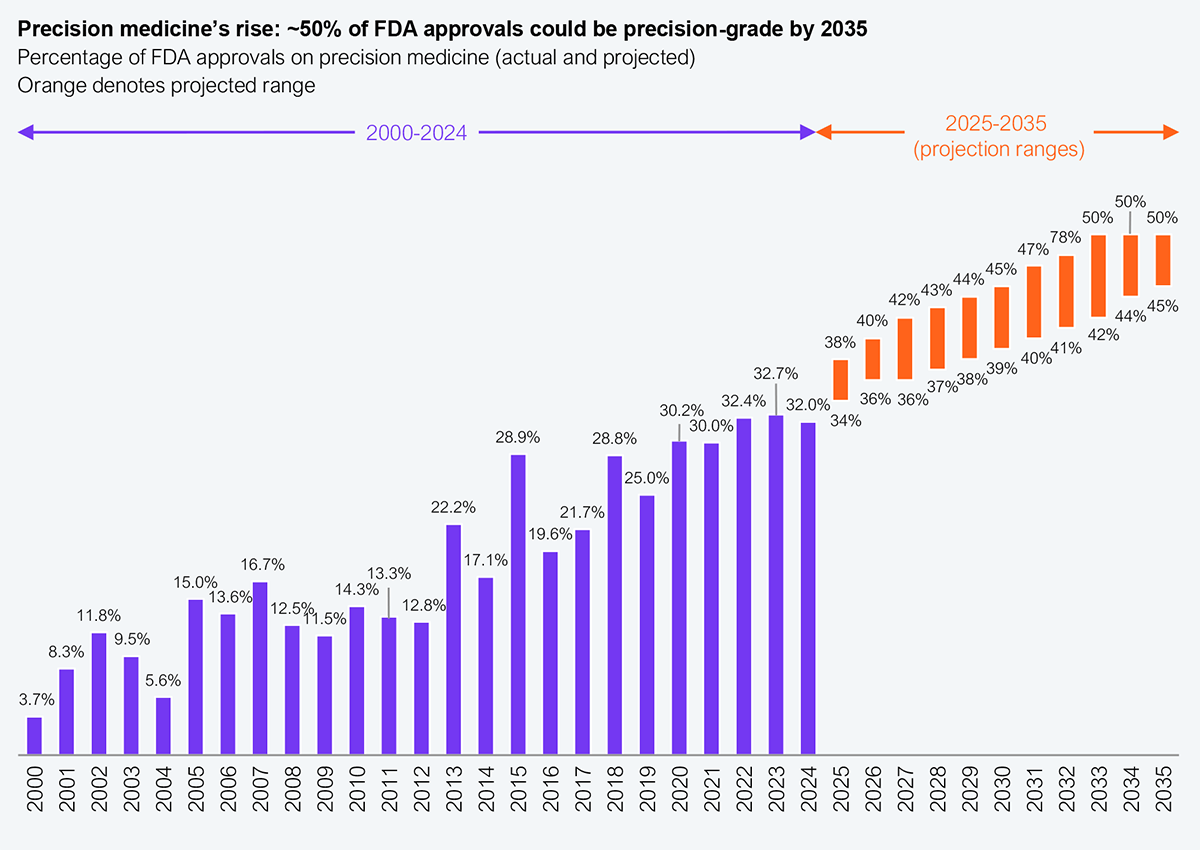
Source: CDRE (FDA); Personalized Medicine Coalition; HFS Research, 2025
The traditional one-size-fits-all model produces inconsistent, often poor outcomes: some patients benefit, while others don’t, and many suffer avoidable side effects. According to data from PubMed and Embase, about 35–44% of patients don’t respond to their initial therapy, and some experience adverse reactions due to genetic differences.
In contrast, precision medicine offers an alternative: using a patient’s genetic, molecular, and lifestyle data to deliver tailored treatments and more optimized outcomes. AI is key to decoding this data and making precision scalable, not just for rare conditions but also across oncology, neurodegeneration, autoimmune disorders, and even chronic disease. For example, IBM Watson for Oncology (WFO) analyzes patient records and generates personalized cancer treatment based on genetic and clinical profiles, already supporting thousands of cases in China with rapid decision support.
The use of AI across drug discovery, trial studies, and analytics will facilitate greater output from precision drugs in the future. Going from “mass” to “personalize,” its impacts will span:
While AI has transformative possibilities in precision medicine, making that a reality goes beyond installing new algorithms. Tailoring pharmaceutical production and supply comes with its own set of challenges, ranging from data environment hurdles to business process issues and increased regulatory scrutiny. To thrive in this space, pharma should also focus on:
What patients experience today is guesswork, and what they expect tomorrow is therapy informed by their biology, not population averages. The following exhibit illustrates how precision medicine actually delivers.
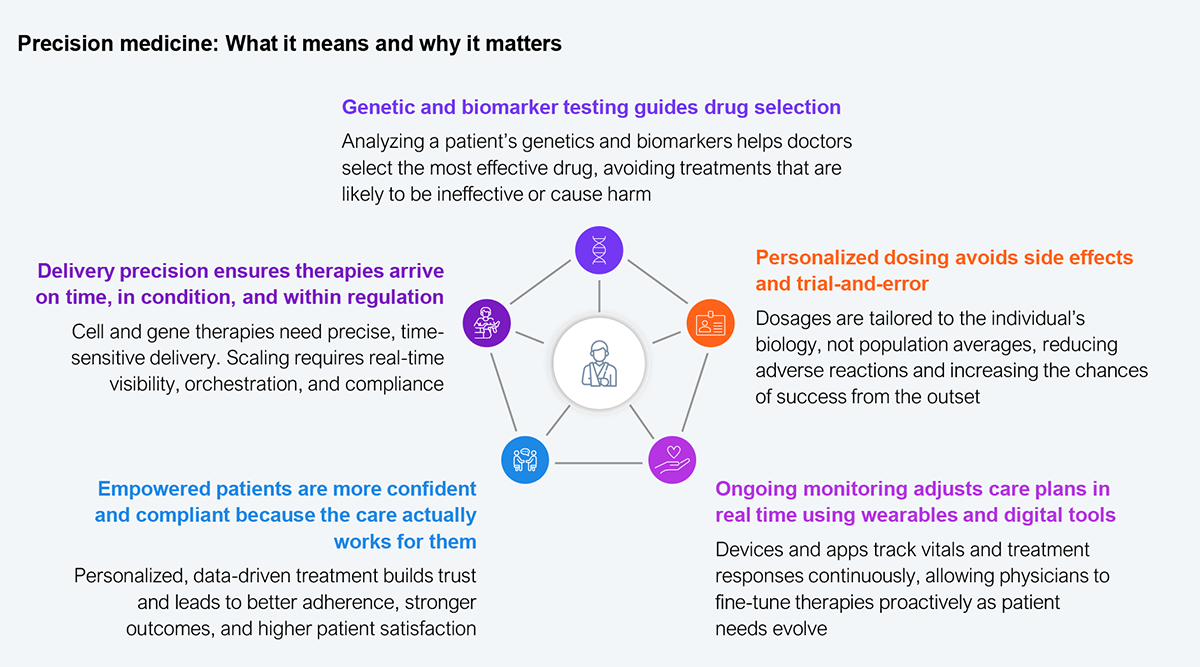
Source: HFS Research, 2025
Traditional drug development produces variable results, whereas precision medicine offers a reliable impact. However, scaling precision requires appropriate data infrastructure, manufacturing preparedness, and regulatory alignment. The future will favor firms that industrialize AI, upgrade infrastructure, and reconfigure themselves for large-scale personalization.
Register now for immediate access of HFS' research, data and forward looking trends.
Get StartedIf you don't have an account, Register here |
Register now for immediate access of HFS' research, data and forward looking trends.
Get Started